buy keytruda online | buy pembrolizumab | keytruda buy hong kong
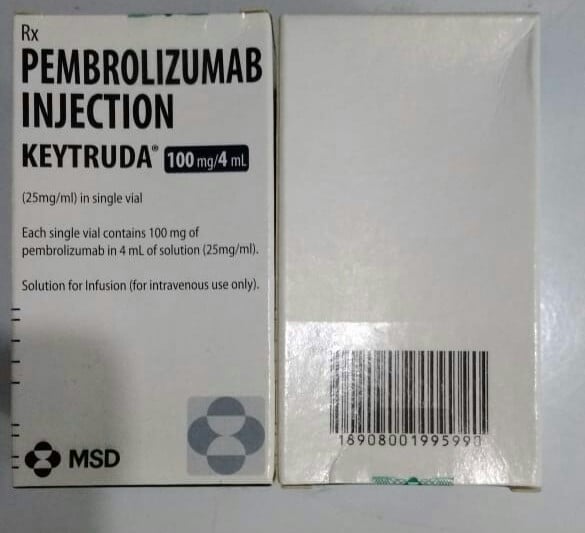
Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, and stomach cancer. It is given by slow injection into a vein.
Common side effects include fatigue, musculoskeletal pain, decreased appetite, itchy skin (pruritus), diarrhea, nausea, rash, fever (pyrexia), cough, difficulty breathing (dyspnea), constipation, pain, and abdominal pain. It is an IgG4 isotype antibody that blocks a protective mechanism of cancer cells and thereby, allows the immune system to destroy them. It targets the programmed cell death protein 1 (PD-1) receptor of lymphocytes. It works by targeting the cellular pathway of proteins found on the body’s immune cells and some cancer cells, known as PD-1/PD-L1.
Pembrolizumab was approved for medical use in the United States in 2014. In 2017, the US Food and Drug Administration (FDA) approved it for any unresectable or metastatic solid tumor with certain genetic anomalies (mismatch repair deficiency or microsatellite instability). It is on the World Health Organization’s List of Essential Medicines
Medical uses

Micrograph showing a PD-L1 positive non-small cell lung carcinoma. PD-L1 immunostain
As of 2019, pembrolizumab is used via intravenous infusion to treat inoperable or metastatic melanoma, metastatic non-small cell lung cancer (NSCLC) in certain situations, as a first-line treatment for metastatic bladder cancer in patients who can’t receive cisplatin-based chemotherapy and have high levels of PD-L1, as a second-line treatment for head and neck squamous cell carcinoma (HNSCC), after platinum-based chemotherapy, for the treatment of adult and pediatric patients with refractory classic Hodgkin’s lymphoma (cHL), and recurrent locally advanced or metastatic esophageal squamous cell carcinoma.
buy keytruda online | buy pembrolizumab | keytruda buy hong kong
For NSCLC, pembrolizumab is a first-line treatment if the cancer overexpresses PD-L1, a PD-1 receptor ligand, and the cancer has no mutations in EGFR or in ALK; if chemotherapy has already been administered, then pembrolizumab can be used as a second-line treatment, but if the cancer has EGFR or ALK mutations, agents targeting those mutations should be used first.[3][16] Assessment of PD-L1 expression must be conducted with a validated and approved companion diagnostic.

Pembrolizumab (Pembro) use for treatment of Lung cancer in the first line with or without chemotherapy (Chemo). Median overall survival in months (M). Hazarad Ration (HR). KEYNOTE-024, KEYNOTE-042, KEYNOTE-189, and KEYNOTE-407. Adapted from: First line Immunotherapy for Non-Small Cell Lung Cancer Nasser NJ, Gorenberg M, Agbarya A. Pharmaceuticals (Basel). 2020 Nov 8;13(11):373. https://doi.org/10.3390/ph13110373
In 2017, the U.S. Food and Drug Administration (FDA) approved pembrolizumab for any unresectable or metastatic solid tumor with certain genetic anomalies (mismatch repair deficiency or microsatellite instability). This was the first time the FDA approved a cancer drug based on tumor genetics rather than tissue type or tumor site; therefore, pembrolizumab is a so-called tissue-agnostic drug.
In the European Union, pembrolizumab is indicated for:
buy keytruda online | buy pembrolizumab | keytruda buy hong kong
- the treatment of advanced (unresectable or metastatic) melanoma in adults as monotherapy.
- the adjuvant treatment of adults with Stage III melanoma and lymph node involvement who have undergone complete resection as monotherapy.
- the first-line treatment of metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumours express PD-L1 with a ≥ 50% tumour proportion score (TPS) with no EGFR or ALK positive tumour mutations as monotherapy.
- the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR or ALK positive mutations in combination with pemetrexed and platinum chemotherapy.
- the first-line treatment of metastatic squamous NSCLC in adults in combination with carboplatin and either paclitaxel or nab-paclitaxel.
- the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥ 1% TPS and who have received at least one prior chemotherapy regimen. People with EGFR or ALK positive tumour mutations should also have received targeted therapy before receiving Keytruda as monotherapy.
- the treatment of adults with relapsed or refractory classical Hodgkin lymphoma (cHL) who have failed autologous stem cell transplant (ASCT) and brentuximab vedotin (BV), or who are transplant-ineligible and have failed BV as monotherapy.
- the treatment of locally advanced or metastatic urothelial carcinoma in adults who have received prior platinum-containing chemotherapy as monotherapy..
- the treatment of locally advanced or metastatic urothelial carcinoma in adults who are not eligible for cisplatin-containing chemotherapy and whose tumours express PD L1 with a combined positive score (CPS) ≥ 10 as monotherapy.
- the first-line treatment of metastatic or unresectable recurrent head and neck squamous cell carcinoma (HNSCC) in adults whose tumours express PD-L1 with a CPS ≥ 1 as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy.
- the treatment of recurrent or metastatic HNSCC in adults whose tumours express PD-L1 with a ≥ 50% TPS and progressing on or after platinum-containing chemotherapy as monotherapy.
- the first-line treatment of advanced renal cell carcinoma (RCC) in adults in combination with axitinib.
buy keytruda online | buy pembrolizumab | keytruda buy hong kong